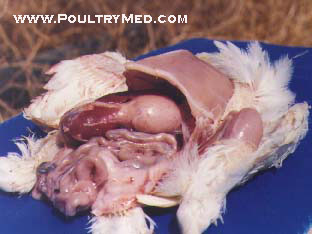
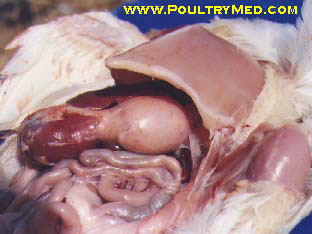
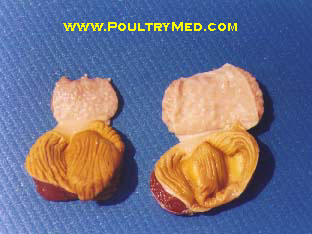
Recently I have encountered an avian case of which we cant really tell what it is cos we're still investigating the case. But from our differential, infectious coryza is one of them. The chicken was found with swollen face plus respiratory problems.
*************************************************************************
Swollen face
Infectious coryza
A usually acute, sometimes chronic, highly infectious disease of chickens, occasionally pheasants and guinea-fowl, characterised by catarrhal inflammation of the upper respiratory tract, especially nasal and sinus mucosae.
Aetiology
The causative bacterium, Haemophilus paragallinarum (gallinarum) is a gram-negative, pleomorphic, nonmotile, catalase-negative, microaerophilic rod that requires nicotinamide adenine dinucleotide (V-factor) for in vitro growth. When grown on blood agar with a staphylococcal nurse colony that excretes the V-factor, the satellite colonies appear as dewdrops, growing adjacent to the nurse colony.
Chocolate agar is an excellent Haemophilus growth medium as it allows for increased accessibility to these factors.Alternatively, Haemophilus is sometimes cultured using the "Staph streak" technique: both Staphylococcus and Haemophilus organisms are cultured together on a single blood agar plate. In this case, Haemophilus colonies will frequently grow in small "satellite" colonies around the larger Staphylococcus colonies because the metabolism of Staphylococcus produces the necessary blood factor by-products required for Haemophilus growth.
Hemophilus on blood agar plate
Transmission
Chronically ill or healthy carrier birds are the reservoir of infection. Chickens of all ages are susceptible, but susceptibility increases with age. The incubation period is 1-3 days, and the disease duration is usually 2-3 wk. Under field conditions, the duration may be longer in the presence of concurrent diseases, eg, mycoplasmosis.
Infected flocks are a constant threat to uninfected flocks. Transmission is by direct contact, airborne droplets, and contamination of drinking water. Commercial farms that have multiple-age flocks tend to perpetuate the disease. Egg transmission does not occur (no vertical transmission).
The bacterium survives 2-3 days outside the bird but is easily killed by heat, drying and disinfectants. Intercurrent respiratory viral and bacterial infections are predisposing factors.
Clinical signs
Facial swelling.
Purulent ocular and nasal discharge.
Swollen wattles.
Sneezing.
Dyspnoea.
Loss in condition.
Drop in egg production of 10-40%.
Inappetance
Facial swelling: Common finding
Purulent discharge. Note the solid white material under the eye
Swelling of infraorbital sinuses and eye discharges
Post-mortem lesions
- Catarrhal inflammation of nasal passages and sinuses.
- Conjunctivitis.
- Eye-lid adherence.
- Caseous material in conjunctiva/sinus.
- Tracheitis.
Severe fibrinosuppurative conjunctivitis
Infraorbital sinus: Catarrhal and suppurative sinusitis
Air sacs: Severe diffuse caseous airsacculitis (uncommon finding)
The histopathologic response of respiratory organs consists of disintegration and hyperplasia of mucosal and glandular epithelia and edema with infiltration of heterophils, macrophages, and mast cells.
Differential diagnosis
- Mycoplasmosis
- Infectious Laryngotracheitis
- Newcastle disease
- Infectious bronchitis
- Avian Influenza
- Swollen head syndrome
- Vitamin A dificiency
Diagnosis
Isolation of a gram-negative, satellitic, catalase-negative organism from chickens in a flock with a history of a rapidly spreading coryza is diagnostic. The catalase test is essential, as nonpathogenic Haemophilus organisms, which are catalase-positive, are present in both healthy and diseased chickens. A PCR test that can be used on the live chicken and that has proved superior to culture, even in developing countries, has been developed. Production of typical signs after inoculation with nasal exudate from infected into susceptible chickens is also reliable diagnostically. No suitable serologic test exists; a hemagglutination-inhibition test is the best of the available tests.
Treatment
Erythromycin and oxytetracycline are usually beneficial. Several new-generation antibiotics (eg, fluoroquinolones, macrolides) are active against infectious coryza. Various sulfonamides, sulfonamide-trimethoprim, and other combinations have been successful but must not be used in layers. In more severe outbreaks, although treatment may result in improvement, the disease may recur when medication is discontinued.
Preventive medication may be combined with a vaccination program, if started pullets are to be reared or housed on infected premises.
Control
Prevention is the only sound method of control. “All-in/all-out” farm programs with sound management and isolation methods are the best way to avoid the disease. Replacements should be raised on the same farm or obtained from clean flocks. If replacement pullets are to be placed on a farm that has a history of infectious coryza, bacterins are available to help prevent and control the disease.
Sources:
The merck's veterinary manual, Altlas of Avian diseases, Cornell university, college of veterinary medicine. Infectious coryza;www.poultrysite.com.