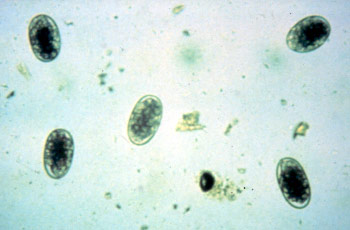
Aim: To check the effectiveness of the anthelmintics by counting the estimate number of the worm's eggs.
Fecal egg count using modified McMaster technique is currently the main measurement used for selection for parasite resistance.
Equipments
- 2 beakers or plastic equipment
- Weighing scale
- Measuring cylinder
- Mortar and pastle
- Filter
- Spatula
- Pipette
- Saturated salt solution
- McMaster slide
- Compound Microscope
- Animal feces
Procedure
1. Weigh 3 grams of faeces and place into a container.
** Feces must be fresh. Wear gloves.
2. Add 45ml saturated salt solution
3. By using mortar and pastle grind off the feces (to remove the fiber) and filter the solution into another beaker.
4. Stir the solution by using spatula and leave for 2-3 minute (to allow the fiber to become sedimentary at the bottom of the beaker
5. Pipette the solution and fill the first compartment of the McMaster slide
** the second compartment may be filled by other sample
for example: 1st compartment = sample A
2nd compartment = sample B
6. Observe the McMaster slide under the compound microscope at 10x10 mag
** Do not use 40x and 100x because the objective will break the thick upper part plate of the McMaster slide.
7. Identify and count all eggs within the area of both chambers
8.The number of eggs per gram can be calculated as follows:
Count the number of eggs within the grid of each chamber, ignoring those outside the squares. For example:
Sample A Sample B
*Read from the first left row down to the second row up to the third row and next..
Calculation
Sample A
12 eggs x 100 epg = 1200 epg
*100 epg is the times factor for the McMaster technique
Sample B
15 eggs x 100 epg = 1500 epg
Interpretation of the results
More than 3000 epg, the animals must be given anthelmintics or change the deworming drugs because the higher the epg numbers indicates the worm's resistance against the anthelmintic drugs.
- Eggs are produced only by fertile adult female (or hermaphrodite) worms and will, therefore, be absent in immature or single sex infections
- The daily output of eggs by fertile females is influenced by host-physiological factors such as stress or lactation ( increased ) or immunity ( decreased )
- Chemotherapy can also affect egg-production e.g. corticosteroids ( increased ) or sub-lethal anthelmintic doses (decreased)
- Some food-stuffs may have a similar effect e.g. tannin-rich forages (decreased )
- The concentration of eggs (per gram of faeces) is influenced by the daily volume of faeces being produced by the host, the rate of passage by the ingesta through the intestine, and the distribution of eggs throughout the faecal mass.
- Some types of eggs are heavier than others and may not float well in solutions of lower specific gravity (e.g. Fasciola)
- Some eggs from different species are indistinguishable (particularly trichostrongylids and strongylids). This complicates clinical interpretation as some species (e.g. Haemonchus) produce many more eggs per day than others (e.g. Ostertagia).
Ruminant eggs (commonly found)
Trichuris ovis
Length 70-80 µm
Width 30-42 µm
Thick-walled
Lemon-shaped with polar plugs
Granular contents, no blastomeres

Nematodirus battus
Length 164 µm
Width 72 µm
Shell thin, brown, parallel sides
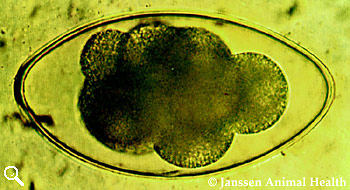
Nematodirus filicollis
Length 150 µm
Width 75 µm
Shell thin, colourless
Nematodirus helvetianus
Length 212 µm
Width 97 µm
Shell thin, colourless
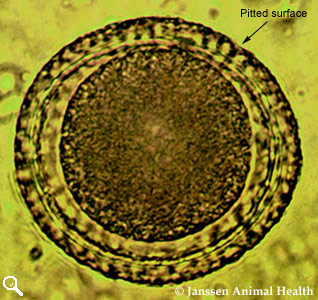
Toxocara vitulorum
Length 69-95 µm
Width 60-77 µm
Subglobular
Thick albuminous shell
Granular contents
Pitted surface to shell
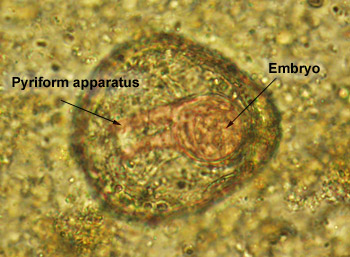
Monezia
(M. expansa and M. benedeni)
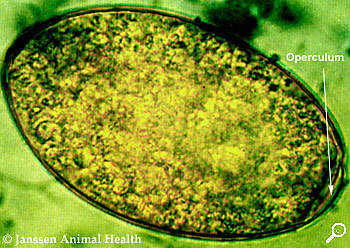
Fasciola Hepatica
Length 130-145 µm
Width 70-90 µm
Regular ellipse
Thin shell
Operculum at one pole
Granular yellowish-brown contents filling whole egg
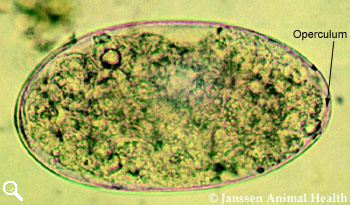
Paramphistomum
Length 160 µm
Width 90 µm
Operculum on one pole
Pale grey to greenish colour

Strongyle
Approximately 80 µm long
Thin-shelled
Broad ellipse
Barrel-shaped side walls
Blastomeres present, number vary
Source: The RVC/FAO Guide to Veterinary Diagnostic Parasitology