This semester takes me on a endless marathon. To get in class on time, with 3 times a week presentation and followup treatment upon every case we encountered. My weekends are not meant to be free.Not to mentioned the subjects I need to head over heel with and struggling to carry out my duty as in educational and social activities.
Owh, I really need a break to catch up. Didn't have much time to write my blog

Tonight (11.16 PM) we will come into one of my case studies- Moraxella bovis (pink eye)
*******************************************************
Pink eye in cattle
Pinkeye (infectious bovine keratoconjunctivitis or New Forest eye) is a common infectious disease affecting the eyes of cattle. The name describes the redness and inflammation of the lining of the eyelid and eyeball. Although pinkeye is non-fatal, it has a marked economic impact on the cattle industry. It is known to occur at all seasons of the year and in all breeds of cattle.
It is a highly contagious disease, causing inflammation of the cornea (the clear outer layer) and conjunctiva (the pink membrane lining the eyelids) of the eye. It will also cause ulceration, which looks like a hole or depression in the cornea. The incidence of pinkeye increases in spring, peaks in the summer, and decreases in the fall. Pinkeye results in mild to severe disease and, in approximately 2 percent of the cases, will cause blindness.

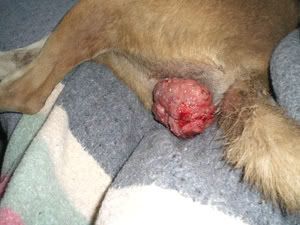
Infected cattle with severe discharge and face flies
Moraxella bovis is the primary infectious agent initiating pinkeye. Other microorganisms initiating pinkeye include Chlamydia, Mycoplasma, and Acholeplasma, or viruses such as the Infectious Bovine Rhinotracheitis (IBR) virus, which can either add to the severity of the disease process or may serve as predisposing factors permitting a secondary infection with M. bovis.Other factors instrumental in causing eye irritation, thereby allowing for invasion of M. bovis and subsequent disease, are excessive ultraviolet light (sunlight), the face fly (Musca autumnalis), the house fly (Musca domestica), the stable fly (Stomoxys calcitrans), plant material, and dust.
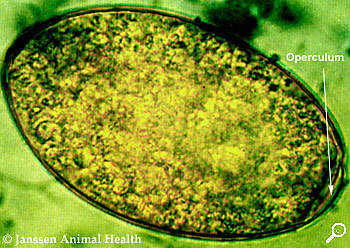
Moraxella bovis is a Gram-negative, aerobic, oxidase-positive diplococcus
Ultraviolet (UV) light is especially a problem for cattle lacking pigmentation around the eye. Lack of pigmentation allows increased UV radiation to sensitize the eye, resulting in inflammation and subsequent infection.Flies not only serve as irritants as they feed on secretions from the eye, but they also serve as a means of transmitting M. bovis from infected to non-infected animals. Face flies can remain infected with M. bovis up to three days following feeding on infected material. Under experimental conditions, disease transmission is uncommon without the presence of face flies and is common with flies present.
Transmission of M. bovis occurs through direct contact, flies, and in-animate objects. The organism is located in the eyes and nasal cavities of infected cattle. Infected secretions from these areas are a source of infection for other cattle. Infected, asymptomatic (no symptoms) cattle may serve as carriers, and will harbor M. bovis in their nasal cavities for a period that may exceed one year. These carrier animals allow for the persistence of pinkeye at a particular site from year to year. Ultraviolet radiation, face flies, growing plants, and pollen production are at their peak in the summer and fall, and account for the high incidence of pinkeye during this period. Weaning distress, increased concentration of cattle, increased exposure to other infectious agents (IBR virus, Mycoplasma, etc.), and hay feeding often are contributing factors to increased disease incidence in late fall, winter, and early spring.
Clinical signs
There are four stages of pinkeye. The disease may resolve at any of these stages while, without treatment, the most severe cases will progress through all four stages.
Stage I: Cattle have excessive tearing and increased sensitivity to light. They will blink frequently and there is redness along the eyelids. Cattle will often seek shade, which will decrease their grazing time. Pain associated with pinkeye also decreases their feed intake. Stage I will progress to a small ulcer in the center of the cornea which appears as a small white spot. The cornea develops a slightly cloudy grey appearance due to inflammation. One or both eyes may be affected.

Stage II: The clinical signs described in Stage I continue, but the ulcer spreads across the cornea. As more inflammation occurs, the cornea becomes increasingly cloudy. At this point, some of the dark color of the iris can still be seen. Blood vessels from the outside portion of the cornea begin to grow across the cornea to help with healing. These blood vessels make the cornea appear pink, which is how the disease received its name.

Stage III: The ulcer covers most of the cornea and the inflammation continues to spread into the inner parts of the eye. When this occurs, the inside of the eye fills with fibrin, which is a pus-like substance that gives the eye a yellow appearance versus the typical brown appearance.

Stage IV: The ulcer extends completely through the cornea, and the iris may protrude through the ulcer. The iris will become stuck in the cornea even after healing. This may lead to glaucoma or persistent swelling of the eye. This eye will be partially or completely blind. The eye may go on to completely rupture, and will develop a shrunken appearance or enlarge if glaucoma (increased eye pressure) is present. This eye will be permanently blind.

Inactive scar: Once healing occurs (except Stage IV) the blood vessels will recede, but the eye may continue to be a cloudy blue color. The blue appearance may eventually resolve and the eye appears clear again. In other cases, depending on the severity of the disease, a white scar may be present even after full resolution of the disease.

Diagnosis
In all species, presumptive diagnosis is based on ocular signs and concurrent systemic disease. It is important to distinguish that the lesions are not due to foreign bodies or parasites. In IBR, upper respiratory signs and conjunctivitis predominate, while keratitis accompanied by ulceration is rare. In bovine malignant catarrhal fever, respiratory signs are prominent with primary uveitis and associated keratitis.
Microbial culture may be beneficial in confirming the causative organisms. Chlamydophila and Mycoplasma spp require special media; the diagnostic laboratory should be consulted prior to sample collection. Cytologic evaluation of stained slides prepared from conjunctival scrapings of sheep and goats may reveal Chlamydophila or Mycoplasma organisms. However, the intracytoplasmic inclusion bodies can be difficult to recognize. PCR analysis can be used to detect Chlamydophila and Mycoplasma spp .
Treatment
Early treatment of cattle with pinkeye is important, not only for a successful outcome of the individual animal affected, but also to stop the shedding of the bacteria to decrease the risk of transmission to other cattle.
Stage I: Long-acting tetracyclines (Biomycin 200®, LA200®, or their generic equivalents) are effective at this stage of infection. The recommended dose is 4.5 cc per 100 pounds of body weight subcutaneously (SQ). A second injection given 48 to 72 hours later may increase the percentage of cattle that responds to treatment. Another option is to inject penicillin and dexamethasone into the bulbar conjunctiva. The bulbar conjunctiva is the thin membrane that covers the white portion (or sclera) of the eye. If the injection is performed correctly, the conjunctiva will swell and a bulge should be seen in this area. A veterinarian, or someone who has been specifically trained by a veterinarian, should perform this procedure. Injections placed in the wrong area are ineffective in treating pinkeye and could damage the eye.
Stage II: Both tetracycline and a bulbar conjunctival injection are administered at the above dosages

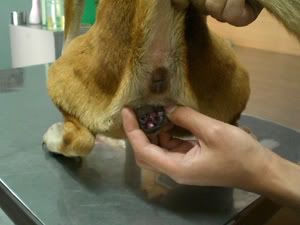
Bulbar conjunctival injection
Stage III: Tetracycline and a bulbar conjunctival injection are administered in conjunction with either an eye patch, suturing the third eyelid over the eye, or suturing the eyelids shut. This makes the eye more comfortable, reducing further irritation, and, therefore, reducing tearing and shedding of the bacteria. Suturing the third eyelid over the eye and suturing the eyelid shut also have the advantage of supporting a fragile cornea to help prevent corneal rupture. Again, this procedure should be done by a veterinarian or someone who has been adequately trained.
Stage IV: Same treatment as Stage III
** In my case, I only use terramycin spray

Prevention
Fly control - continues to be necessary due to isolated areas in with a significant face fly population. Insecticide fly tags, sprays, charged backrubbers, and dusts bags are products that can provide chemical control. Manure, weed, and brush management are necessary for total fly control.
Grass, weed, and brush control - Grazing management, brush beating, mowing, and spraying, minimize pollen and mechanical irritation.
Hay and/or feed bunk management - lower overhead hay feeders, spread hay out, do not feed hay containing mature seed heads or cheat grass in overhead feeders or in round bales, increase bunk space to decrease direct contact.Ultraviolet light (sun light) - breed for eyelid pigmentation, introduce Brahman influence into the herd, provide shade or tree rows with ample room to prevent overcrowding.
Disease management – provide proper immunization against viral diseases (IBR and BVD), isolate infected animals, and decrease environmental and nutritional distress.
Ultraviolet light (sun light) - breed for eyelid pigmentation, introduce Brahman influence into the herd, provide shade or tree rows with ample room to prevent overcrowding.
Sources: Pinkeye in beef cattle:Virginia coorperative extension, Pink eye in cattle Okhlahoma cooperative extension service, The Merck's veterinary manual 10th ed