It's been a while since my last post. I was very busy last week so I dont have much time to update this blog. I'm just coming back from Ipoh, after attending Veterinary Association Malaysia congress and it was awesome. I can imagine myself standing there and present my research paper. Haha. Ok2, back to business!
Just started my pathology class and Prof Imad taught the first things about tissue injury and as well as necrosis.
******************************************************
Types of necrosis
The death of cells in a living tissue is called Necrosis. Necrosis is commonly referred to as a bedsore. The degradation of the tissue starts with swelling within the cell and ultimately an interruption of the membranes of the cell. The organelles begin to breakdown and this spreads and causes an inflammation. Necrosis can be caused due to an injury or infection of some kind. If the infection is not tended to correctly, the chances of necrosis starting is more likely. Necrosis is more severe than other cell deaths in infections because once those cells die they can release chemicals that are harmful or will damage other cells.
example of necrosis: death tissue in LIVING cell
According to Prof Imad, there are 4 major types of necrosis namely; coagulative, liquefaction, caseous, and fat necrosis.
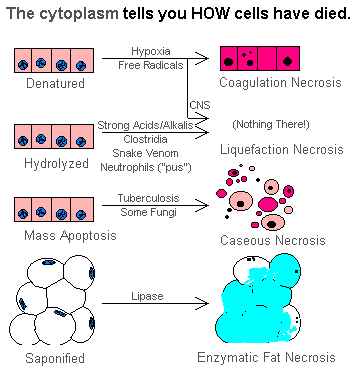
1. Coagulative necrosis

Coagulative: Caused by ischemia. Ischemia results in decreased ATP, increased cytosolic Ca++, and free radical formation, which each eventually cause membrane damage.
- Decreased ATP results in increased anaerobic glycolysis, accumulation of lactic acid, and therefore decreased intracellular pH.
- Decreased ATP causes decreased action of Na+ / K+ pumps in the cell membranes, leading to increased Na+ and water within the cell (cell swelling).
- Other changes: Ribosomal detachment from endoplasmic reticulum; blebs on cell membranes, swelling of endoplasmic reticulum and mitochondria.
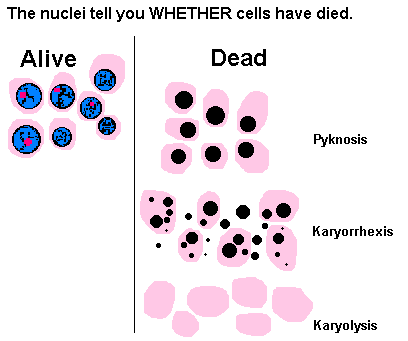
- Up to here, the changes are reversible if oxygenation is restored by reversing the ischemia. If the ischemia continues, necrosis results, causing the cytoplasm to become eosinophilic, the nuclei to lyse or fragment or become pyknotic (hyperchromatic and shrunken). In the early stages of necrosis, the cells remain for several days as ghosts of their former selves, allowing one to still identify them and the tissue (in contrast to the other types of necrosis). The cellular reaction is polys, followed by a granulation tissue responses.

Coagulative necrosis
Characterised by preservation of tissue achitecture, increased cytoplasmic eosinophilia and nuclear changes. The cell may look pyknosis (chromatin clumping and shrinking: increased eosinophilia), karyorrhexis (fragmentation of chromatin), karyolysis (fading of chromatin material) and dissapearance of stainable nuclei
2. Liquefactive necrosis

Usually caused by focal bacterial infections, because they can attract polymorphonuclear leukocytes. The enzymes in the polys are released to fight the bacteria, but also dissolve the tissues nearby, causing an accumulation of pus, effectively liquefying the tissue (hence, the term liquefactive).
The result of hydrolysis. When the cells die, they are rapidly destroyed by lysosomal enzymes, either their own or those from neutrophilic leukocytes (i.e., bacterial infections), or clostridia or snake poison. Acid and lye burns represent the extreme of liquefaction. Also, if both neurons and glia are killed, dead brain liquefies rapidly.
Liquefactive necrosis that is caused by neurophilic leukocytes is called PUS. The term may also be used for an effusion that is full of dead neutrophils. Because of the extensive protein hydrolysis (i.e., more total molecules), there's a tremendous increase in the osmotic pull. This explains the familiar high-pressure in a ripened pimple -- and deep in the brain, for example, this is even more serious.
NOTE: The truism that "brain liquefies" is a common source of misunderstanding. Brain deprived of its oxygen for a few moments will suffer neuronal damage but not necrosis. Brain deprived of blood flow for a few moments longer will lose neuronal structure but not glia, and remain solid. The same is true of diseases in which neurons die off one at a time (i.e., Alzheimer's disease causes the brain to shrivel but not to liquefy). Only if the glia are killed does the brain melt away, and then only after several days.
Liquefactive necrosis in the brain

liquefactive necrosis of the brain demonstrates many macrophages at the right which are cleaning up the necrotic cellular debris.
3. Caseous necrosis
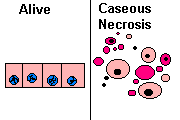
All of the cells in an area die, the tissue architecture is obliterated, and they turn into a crumbly ("friable"), readily-aerosolized powder.
A distinct form of coagulative necrosis seen in mycobacterial infections (e.g., tuberculosis), or in tumor necrosis, in which the coagulated tissue no longer resembles the cells, but is in chunks of unrecognizable debris. Usually there is a giant cell and granulomatous reaction, sometimes with polys, making the appearance distinctive.
Gross appearance of caseous necrosis in a hilar lymph node infected with tuberculosis. The node has a cheesy tan to white appearance. Caseous necrosis is really just a combination of coagulative and liquefactive necrosis that is most characteristic of granulomatous inflammation.

Found in granulomatous inflammation; manifestation of partial immunity of interaction T-Lymphocyte, macrophage, and cytokines associated with tubercolosis. Architecture is not preserved but tissue is not liquefied. Grossly soft and cheeselike. Histologically amorphous and acidophilic.
4. Fat necrosis

A term for necrosis in fat, caused either by release of pancreatic enzymes from pancreas or gut (enzymic fat necrosis) or by trauma to fat, either by a physical blow or by surgery (traumatic fat necrosis). The effect of the enzymes (lipases) is to release free fatty acids, which then can combine with calcium to produce detergents (soapy deposits in the tissues). Histologically, one sees shadowy outlines of fat cells (like coagulative necrosis), but with Ca++ deposits, foam cells, and a surrounding inflammatory reaction.
This is fat necrosis of the pancreas. Cellular injury to the pancreatic acini leads to release of powerful enzymes which damage fat by the production of soaps, and these appear grossly as the soft, chalky white areas seen here on the cut surfaces.
Fat necrosis adjacent to pancreas is seen here. There are some remaining steatocytes at the left which are not necrotic. The necrotic fat cells at the righthave vague cellular outlines, have lost their peripheral nuclei, and their cytoplasm has become a pink amorphous mass of necrotic material.
Other types of necrosis
5. Gangrenous necrosis
Due to cut off blood supply to lower extremities or bowel due to vascular occlusion.
GANGRENE ("gangrenous necrosis") is not a separate kind of necrosis at all, but a term for necrosis that is advanced and visible grossly. If there's mostly coagulation necrosis, (i.e., the typical blackening, desiccating foot that dried up before the bacteria could overgrow), we call it DRY GANGRENE. If there's mostly liquefactive necrosis (i.e., the typical foul-smelling, oozing foot infected with several different kinds of bacteria), or if it's in a wet body cavity, we call it WET GANGRENE.
Occur to most diabetic patients
Gangrenous necrosis at lower limb (dry gangrene)
wet gangrene (bacterial rich)
6. Fibrinoid necrosis
Due to the deposition of fibrin-like material on arterial walls due to immune-mediated vasculitis.
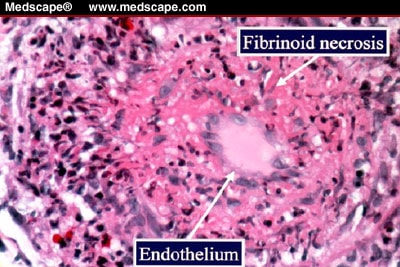
Fibroid necrosis: smudgy pink material in vascular walla with or without necrosis
Sources: USMLE wiki: Cell pathology; cell injury and death Ed Friedlander M.D pathologist; cell injury and necrosis www.uvm.edu; cell injury, library.med.utah.edu





















