Just finished up my field work with few of my colleagues doing some investigation in farms around Bachok, Kelantan. As we had had encountered few cases involving various types of ruminants, I was assigned to present fiver fluke manifestation in ruminants particularly in cattle which are commonly found there.
*********************************************************

F. Hepatica
Fasciola hepatica is the species of parasitic flatworms that infects the liver of various mammals, including humans. The disease caused by the organism is called Fascioliasis or Fasciolosis. It is a trematode species of phylum platyhelminthes. F. hepatica is distributed worldwide, and causes great economic losses in sheep and cattle. It has been known as an important parasite of sheep and cattle for hundreds of years.
Susceptible animal hosts for Fasciola species include:Domestic animals: cattle, sheep, pigs, buffaloes and donkeys;Other domestic animals: horses, goats, dromedaries, camels and llamas; (hares, rabbits and rodents).
F. Hepatica in the liver of infected animal
Morphology
Fasciola hepatica is one of the largest flukes of the world, reaching a length of 30mm and a width of 13mm. It is leaf shaped, pointed posteriorly and wide anteriorly, although the shape varies somewhat. The oral sucker is small but powerful and is located at the end of a cone-shaped projection at the anterior end. The acetabulum is larger than the oral sucker and is anterior. The tegument is covered with large, and scalelike spines.
The intestinal ceca are highly dendritic and extend to near the posterior end of the body. The testes are large and greatly branched, arranged in tandem behind the ovary. The smaller, dendritic ovary lies on the right side, coiling between the ovary and the preacetabular cirrus pouch. Vitelline follicles are extensive, filling most of the lateral body and becoming confluent behind the testes.

F. Hepatica
Life cycle

Life cycle of F.hepatica
The shape of the parasite looks like a leaf, with one end tapering. Each individual parasite posseses ovaries and testes, making them hermaphroditic and able to produce fertilized eggs. The organism attaches to bile ducts with two powerful suckers on each end of its body. The bile ducts are where the mature flukes and release eggs. The eggs are passed out in the host's feces. At temperatures over 10 degrees Celcius, the eggs hatch and release miracidiae within two weeks. The hatchlings must find a Lymanaea snail host within 24 hours of hatching or they will die.
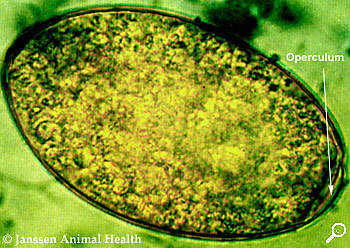
F. Hepatica egg under microscope magnification
After identifying a host snail, the hatchling Fasciola heptica burrow in and begin developing into sporocyst. The germinal cells of the sporocyst develop into rediae which migrate to the hepato-pancreas of the snail. The rediae then develop into cercariae which pass through the snail into water. Cercariae encyst apon nearby plant matter for several months, where they can be ingested by a mammal. Once a mammal ingests these metacercariae, the pH levels of the animals stomach allow the Metacercariae to lose their shell. The cercariae freed from their cyst capsules, burrow through the stomach lining and find their way to the liver. The immature flukes feed on the liver tissue for 6 to 8 weeks, causing the symptoms of acute infection. At this stage the flukes move into the bile ducts where they mature and release eggs, completing it's life cycle. Adult females can produce up to 25000 per day.
Lymnea truncatula
Intermediate host for F.Hepatica larval stage

Clinical signs
Fasciolosis ranges in severity from a devastating disease in sheep to an asymptomatic infection in cattle. The course usually is determined by the number of metacercariae ingested over a short period. In sheep, acute fasciolosis occurs seasonally and is manifest by a distended, painful abdomen; anemia; and sudden death. Deaths can occur within 6 wk of infection. The acute syndrome must be differentiated from “black disease.”
In subacute disease, survival is longer (7-10 wk), even in cases with significant hepatic damage, but deaths occur due to hemorrhage and anemia. Chronic fasciolosis is seen in all seasons; signs include anemia, unthriftiness, submandibular edema, and reduced milk secretion, but even heavily infected cattle may show no clinical signs. Heavy chronic infection is fatal in sheep.Sheep do not appear to develop resistance to infection, and chronic liver damage is cumulative over several years. In cattle, there is evidence of reduced susceptibility after fibrosis of liver tissues and calcification of bile ducts.
Bottle jaw (Submandibular edema) in the infected cattle
In human**
The acute phase of infection in humans is characterized by the migration of immature worms through the liver; hemorrhage and inflammation of the liver can be severe, including fever, abdominal pain, respiratory disturbances and skin rashes. The chronic phase starts when the worms move into the bile ducts; symptoms are nonspecific and usually mild. However, progressive inflammation, leads to fibrosis and thickening of the walls of the ducts and gallbladder that eventually may result in blockage due to the parasites, or their debris. In this case, abdomen pain is a characteristic symptom. Chronic infections can lead to biliary cirrhosis with scarring and fibrosis of the liver and growth deficiencies.
Lesions
Immature, wandering flukes destroy liver tissue and cause hemorrhage. In acute fasciolosis, damage is extensive; the liver is enlarged and friable with fibrinous deposits on the capsule. Migratory tracts can be seen, and the surface has an uneven appearance. In chronic cases, cirrhosis develops. Mature flukes damage the bile ducts, which become enlarged, or even cystic, and have thickened, fibrosed walls. In cattle, the duct walls become greatly thickened and often calcified. Flukes may be found in aberrant sites, eg, lungs. Mixed infections with Fasciola magna can be seen in cattle.
Tissue destruction by wandering flukes may create a microenvironment favorable to activation of clostridial spores. (C.Noyvi type B- Black disease)
Little damage is done by juveniles penetrating the intestinal wall and the capsule surrounding the liver but much necrosis results from migration of flukes through the liver parenchyma. During this time, they feed on liver cells and blood. Anemia sometimes results from heavy infections. Worms in bile ducts cause inflammation and edema, which in turn stimulate production of fibrous tissue in the walls of these ducts. Thus thickened, the ducts can handle less bile and are less responsive to needs of the liver. Back pressure causes atrophy of liver parenchyma, with concomitant cirrhosis and possibly jaundice. In heavy infections the gall bladder is damaged, and walls of the bile ducts are eroded completely.
Fibrous liver tissue
Diagnosis
The oval, operculated, golden brown eggs, 130-150 × 65-90 µm, must be distinguished from those of paramphistomes (rumen flukes), which are larger and clear. Eggs of F hepatica cannot be demonstrated in feces during acute fasciolosis. In subacute or chronic disease in cattle, the number varies from day to day, and repeated fecal examination may be required.
Diagnosis can be aided by an ELISA (commercially available in Europe) that enables diagnosis ~2-3 wk after infection and well before the prepatent period. Plasma concentrations of γ-glutamyltransferase, which are increased with bile duct damage, are also helpful during the late maturation period when flukes are in the bile ducts. At necropsy, the nature of the liver damage is diagnostic. Adult flukes are readily seen in the bile ducts, and immature stages may be squeezed or teased from the cut surface.
Drug of choice: Bithionol or Triclabendazole
Note: Praziquantel, a common drug for treating trematode infections is not very effective against Fasciola hepatica.
Prevention and control
Control measures for F hepatica ideally should involve removal of flukes in affected animals, reduction of the intermediate host snail population, and prevention of livestock access to snail-infested pasture. In practice, only the first of these is used in most cases.
While molluscicides can be used to reduce lymnaeid snail populations, those that are available all have drawbacks that restrict their use. Copper sulfate, if applied before the snail population multiplies each year, is effective but toxic to sheep, which must be kept off treated pasture for 6 week after application. Prevention of livestock access to snail-infested pasture is frequently impractical because of the size of the areas involved and the consequent expense of erecting adequate fencing.
Sources: Merck Veterinary Manual, Wikipedia: F. Hepatica, F.Hepatica, IVD research.com




















3 comments:
While I agree for nationalization of the healthcare services, the root cause is that we have limited the number of doctors that we produce in our country. best liver transplant hospital in india
I appreciate you sharing this information with me.
Every urological issue can be resolved here at the best urology hospital in Chennai . Modern, fully equipped operating rooms provide you with comprehensive patient care.
Post a Comment