- 2 beakers or plastic equipment
- Weighing scale
- Measuring cylinder
- Mortar and pastle
- Filter
- Spatula
- Pipette
- Saturated salt solution
- McMaster slide
- Compound Microscope
- Animal feces






8.The number of eggs per gram can be calculated as follows:

- Eggs are produced only by fertile adult female (or hermaphrodite) worms and will, therefore, be absent in immature or single sex infections
- The daily output of eggs by fertile females is influenced by host-physiological factors such as stress or lactation ( increased ) or immunity ( decreased )
- Chemotherapy can also affect egg-production e.g. corticosteroids ( increased ) or sub-lethal anthelmintic doses (decreased)
- Some food-stuffs may have a similar effect e.g. tannin-rich forages (decreased )
- The concentration of eggs (per gram of faeces) is influenced by the daily volume of faeces being produced by the host, the rate of passage by the ingesta through the intestine, and the distribution of eggs throughout the faecal mass.
- Some types of eggs are heavier than others and may not float well in solutions of lower specific gravity (e.g. Fasciola)
- Some eggs from different species are indistinguishable (particularly trichostrongylids and strongylids). This complicates clinical interpretation as some species (e.g. Haemonchus) produce many more eggs per day than others (e.g. Ostertagia).

Trichuris ovis
Length 70-80 µm
Width 30-42 µm
Thick-walled
Lemon-shaped with polar plugs
Granular contents, no blastomeres

Nematodirus battus
Length 164 µm
Width 72 µm
Shell thin, brown, parallel sides
Nematodirus filicollis
Length 150 µm
Width 75 µm
Shell thin, colourless
Nematodirus helvetianus
Length 212 µm
Width 97 µm
Shell thin, colourless
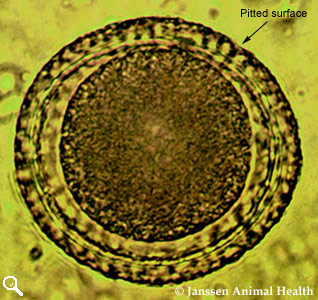
Toxocara vitulorum
Length 69-95 µm
Width 60-77 µm
Subglobular
Thick albuminous shell
Granular contents
Pitted surface to shell
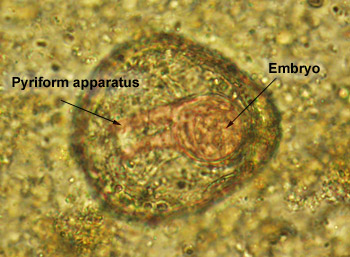
Monezia
(M. expansa and M. benedeni)
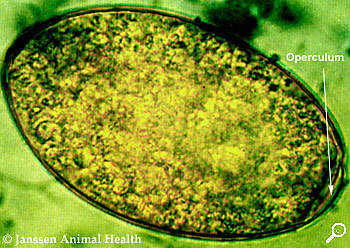
Fasciola Hepatica
Length 130-145 µm
Width 70-90 µm
Regular ellipse
Thin shell
Operculum at one pole
Granular yellowish-brown contents filling whole egg
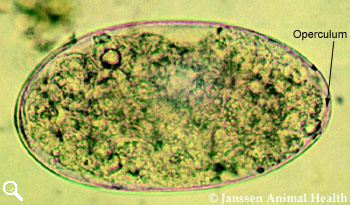
Paramphistomum
Length 160 µm
Width 90 µm
Operculum on one pole
Pale grey to greenish colour
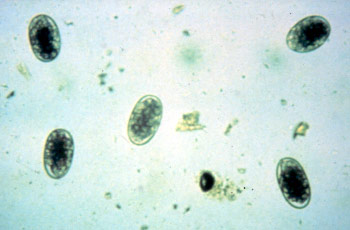
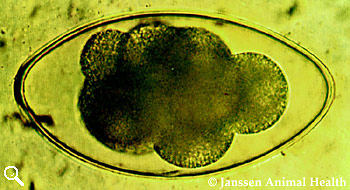
Strongyle
Approximately 80 µm long
Thin-shelled
Broad ellipse
Barrel-shaped side walls
Blastomeres present, number vary
Source: The RVC/FAO Guide to Veterinary Diagnostic Parasitology





















0 comments:
Post a Comment